




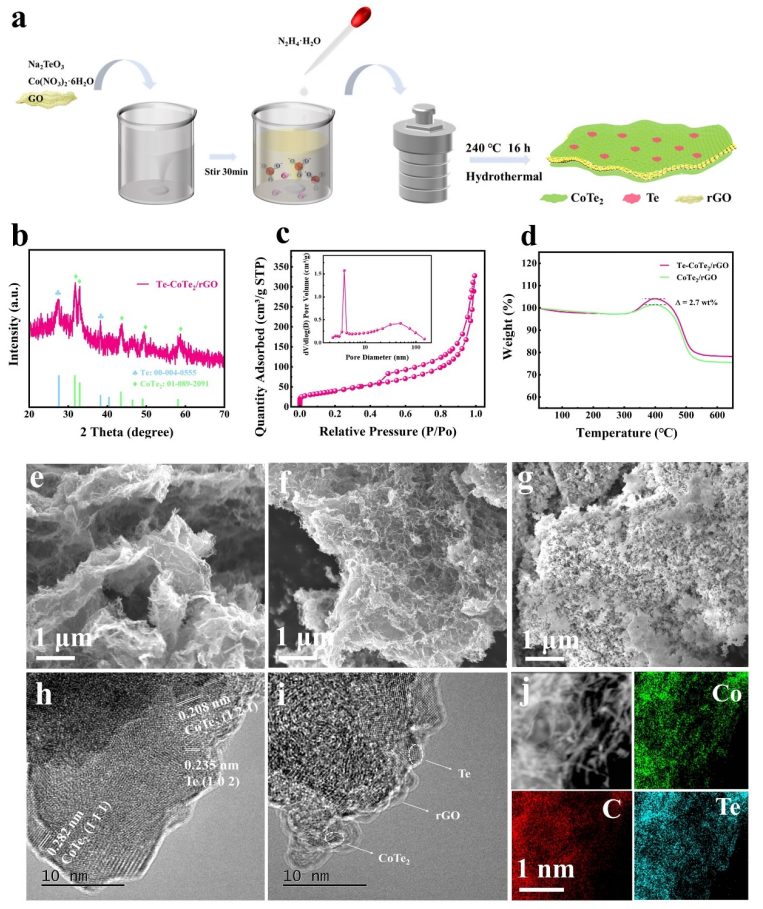
Fig. 1. (a) Schematic diagram of the sample synthesis process. (b) The XRD pattern of Te-CoTe2/rGO, (c) nitrogen adsorption-desorption isotherm and the pore size distribution (inset) of Te-CoTe2/rGO, (d) TGA curves of Te-CoTe2/rGO and CoTe2/rGO in air. SEM images of (e) Te-CoTe2/rGO, (f)CoTe2/rGO and (g) Te-CoTe2. (h, i) HRTEM images of Te-CoTe2/rGO. (j) Mapping of Te-CoTe2/rGO.
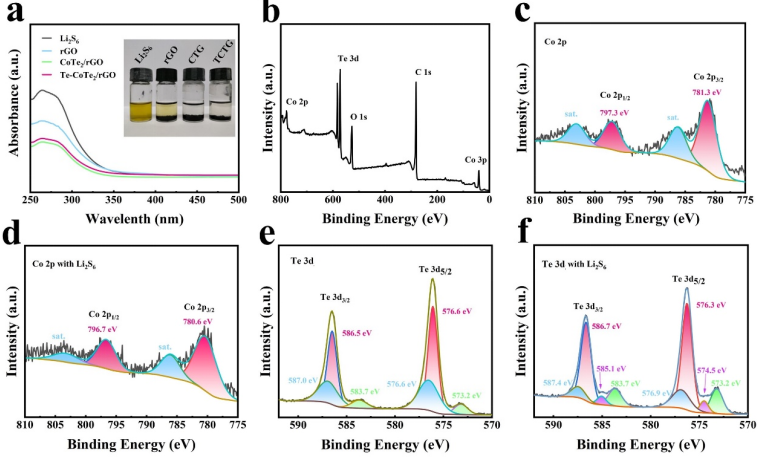
Fig. 2. (a) UV?vis spectra and photo (inside) of rGO, CoTe2/rGO (CTG), and Te-CoTe2/rGO (TCTG) after adsorption of Li2S6 solutions. XPS survey spectrum of Te-CoTe2/rGO (b) and elemental high-resolution XPS spectra of Co 2p before (c) and after (d) absorbing Li2S6 and Te 3d before (e) and after (f) absorbing Li2S6.
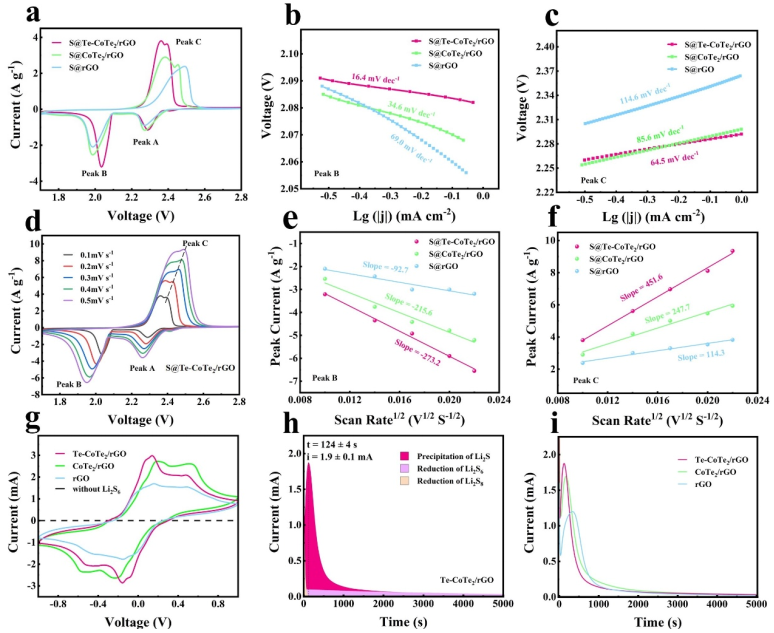
Fig. 3. (a) CV curves of S@Te-CoTe2/rGO, S@CoTe2/rGO and S@rGO cathodes at 0.1 mV s-1. The corresponding Tafel plots of (b) peak B in the reduction direction and (c) peak C in the oxidization direction. (d) CV curves of the S@Te-CoTe2/rGO cathode at different scan rates. (e, f) The corresponding linear fitting curves of the peak currents from 0.1 mV s-1 to 0.5 mV s-1. (g) CV curves of symmetric cells using Te-CoTe2/rGO, CoTe2/rGO and rGO electrodes with or without Li2S6 at a scan rate of 1 mV s-1. Potentiostatic nucleation curves of Li2S with the (h) Te-CoTe2/rGO electrode and (i) Te-CoTe2/rGO, CoTe2/rGO and rGO electrodes in the Li2S8 catholyte.
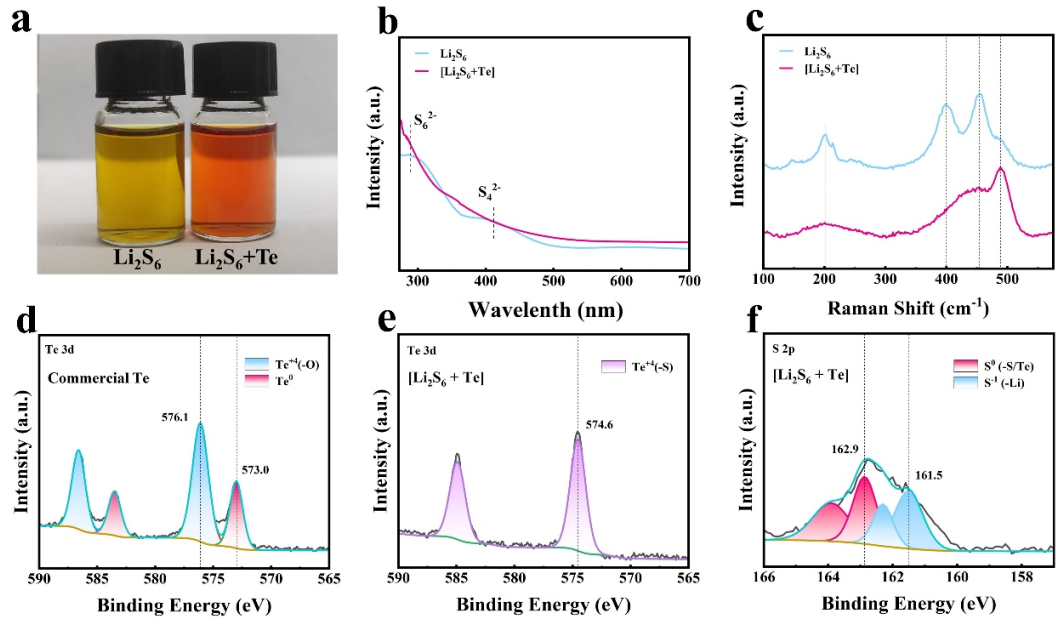
Fig. 4. (a) Comparison between Li2S6 solution and [Li2S6 + Te] solution. (b) UV?vis spectra of Li2S6 and [Li2S6 + Te]. (c) Raman spectra of Li2S6 and [Li2S6 + Te]. XPS spectra: (d) Te 3d of commercial Te, (e) Te 3d of [Li2S6 + Te], (f) S 2p of [Li2S6 + Te].

Fig. 5. (a) CE of the Li‖Cu half cells with and without (w/o) Li2TexSy (LTS). (b, c) The corresponding selected voltage profiles. (d, e) Cycling stability of the Li‖Li symmetric cells with and without Li2TexSy. (f) Rate performances of the Li‖Li symmetric cells with Li2TexSy at different current densities. Surface morphology characterizations of (g) Li sheet before cycling, (h) Li sheet with Li2TexSy and (i) Li sheet without Li2TexSy after 100 cycles at 1 mA cm-2 for 1 mA h cm-2.

Fig. 6. (a) Rate performances and (b) the corresponding charge?discharge profiles of S@Te-CoTe2/rGO, S@CoTe2/rGO and S@rGO cathodes at 0.2 C. (c) Charge?discharge profiles of the S@Te-CoTe2/rGO cathode with different current densities. EIS spectra (d) before and (e) after 100 cycles. (f) Cycling performances of S@Te-CoTe2/rGO cathode at 0.5 C, 1 C and 2 C. (g) Cycling performance of the S@Te-CoTe2/rGO cathode under high sulfur loading. (h) Long cycling performances of S@Te-CoTe2/rGO and CoTe2/rGO cathodes.
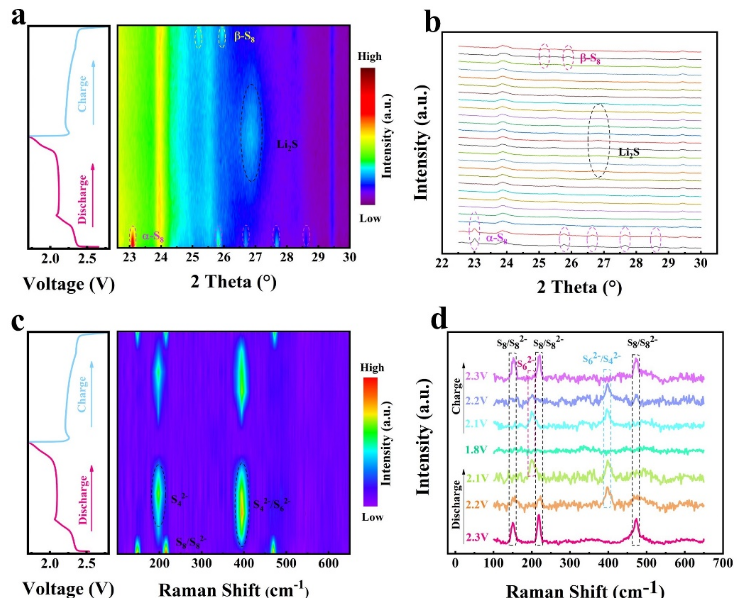
Fig. 7. (a, b) In situ XRD patterns and (c, d) in situ Raman curves of the S@Te-CoTe2/rGO cathode.
Z. Cheng, M. Wang, Y. Dong, Y. Han, X. Yan, L. Xie, X. Zheng, L. Han, J. Zhang, Two-birds with one stone: Improving both cathode and anode electrochemical performances via two-dimensional Te-CoTe2/rGO ultrathin nanosheets as sulfur hosts in lithium-sulfur batteries, Journal of Colloid and Interface Science (2023), doi: https://doi.org/10.1016/j.jcis.2023.06.037
聲明:化學加刊發(fā)或者轉(zhuǎn)載此文只是出于傳遞、分享更多信息之目的,并不意味認同其觀點或證實其描述。若有來源標注錯誤或侵犯了您的合法權益,請作者持權屬證明與本網(wǎng)聯(lián)系,我們將及時更正、刪除,謝謝。 電話:18676881059,郵箱:gongjian@huaxuejia.cn